Abstract
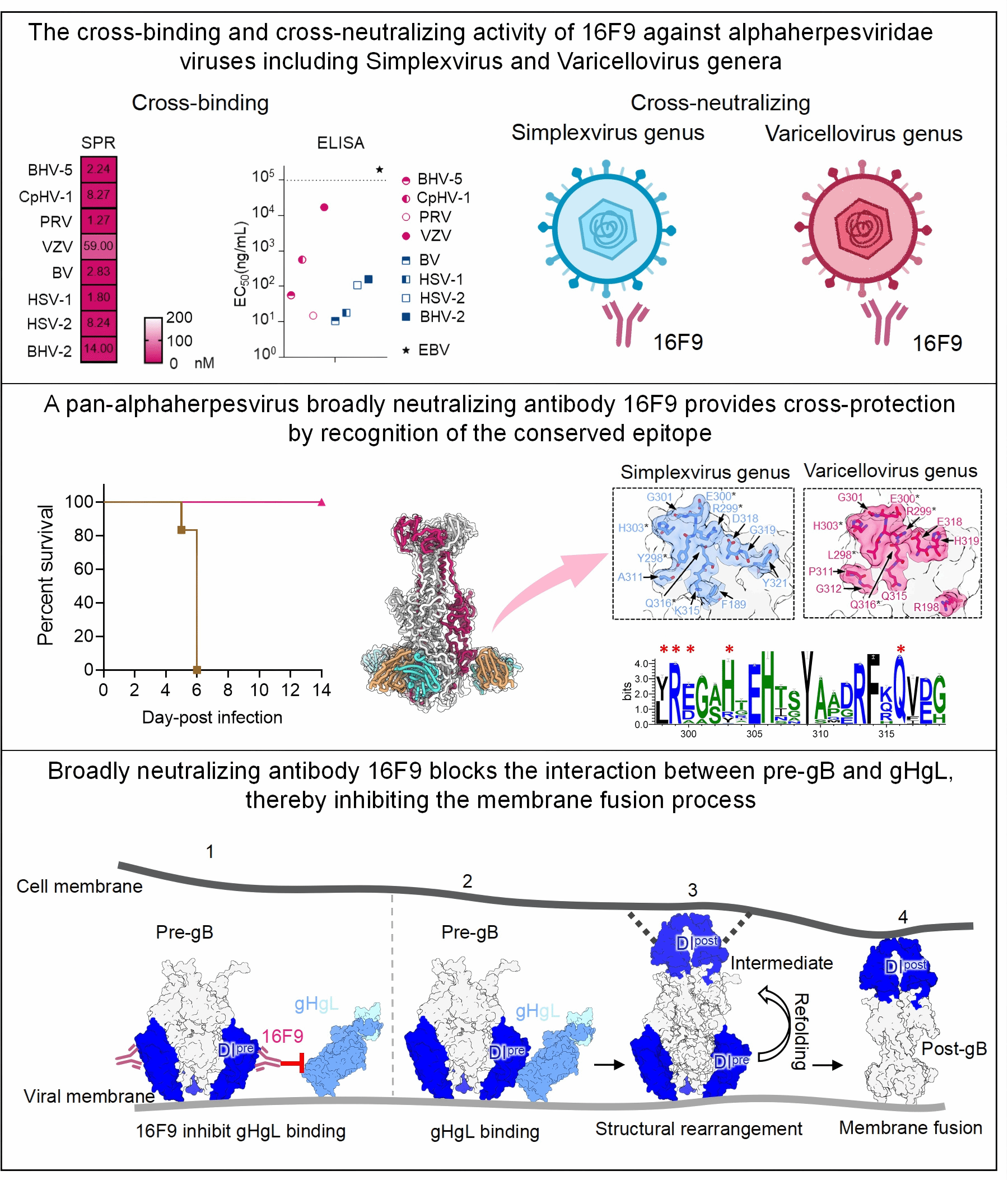
The global prevalence and disease burden of alphaherpesviruses infections, including human-infecting viruses such as HSV-1, HSV-2, and VZV, as well as animal-infecting viruses like PRV, BHV, CHV, and FHV, highlight the unmet need for more effective and universal antiviral strategies. However, there has been no significant progress in developing broad-spectrum interventions against herpesvirus. Here we report the identification of a broadly neutralizing antibody against alphaherpesviruses, 16F9, which targets the glycoprotein B (gB) of alphaherpesviruses and offers cross-protection against multiple viruses such as HSV-1, HSV-2, and PRV in mice. 16F9 demonstrated robust therapeutic efficacy in various female mouse models of herpesvirus diseases including PRV-induced viral encephalitis, HSV-1-induced viral encephalitis, viral keratitis, cutaneous herpes, and neonatal herpesvirus infections. High-resolution cryo-electron microscopy structures revealed that 16F9 binds a conserved site of vulnerability on Domain I of gB. The binding of 16F9 disrupts the interaction between pre-gB and gHgL complex, thereby preventing viral membrane fusion and blocking viral infection. This study provides a foundation for advancing antiviral strategies and underscores the potential of gB-targeted interventions for combating herpesvirus infections.
Link:https://www.nature.com/articles/s41467-025-66099-8