Summary
Background
A safe and highly efficacious Escherichia coli (E. coli)-produced HPV 16/18 bivalent vaccine has beenprequalified by the World Health Organization. Here, we conducted a single-center, open-label, dose-escalation phase1 clinical trial to evaluate the safety and immunogenicity of the second-generation nonavalent HPV 6/11/16/18/31/33/45/52/58 vaccine.
Method
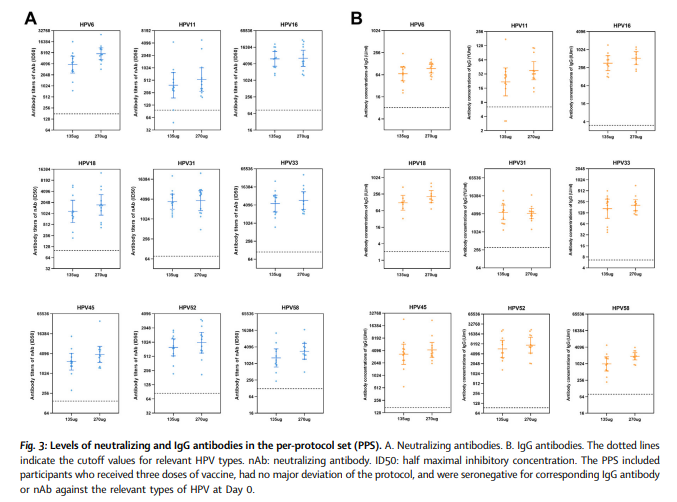
Twenty-four eligible volunteers aged 18–45 years were enrolled in January 2019 in Dongtai, China andreceived 0.5 mL (135 μg) or 1.0 mL (270 μg) of the candidate vaccine with a 0/1/6-month dose-escalation schedule.Local and systemic adverse events (AEs) occurring within 30 days after each vaccination and serious adverse events(SAEs) occurring within 7 months were recorded. Blood samples from each participant were collected before and 2days after the first and third vaccinations to determine changes in laboratory parameters. Serum IgG and neutralizingantibody (nAb) levels against each HPV type at month 7 were analyzed (ClinicalTrials.gov: NCT03813940).
link:https://www.thelancet.com/journals/lanwpc/article/PIIS2666-6065(23)00049-4/fulltext