Abstract
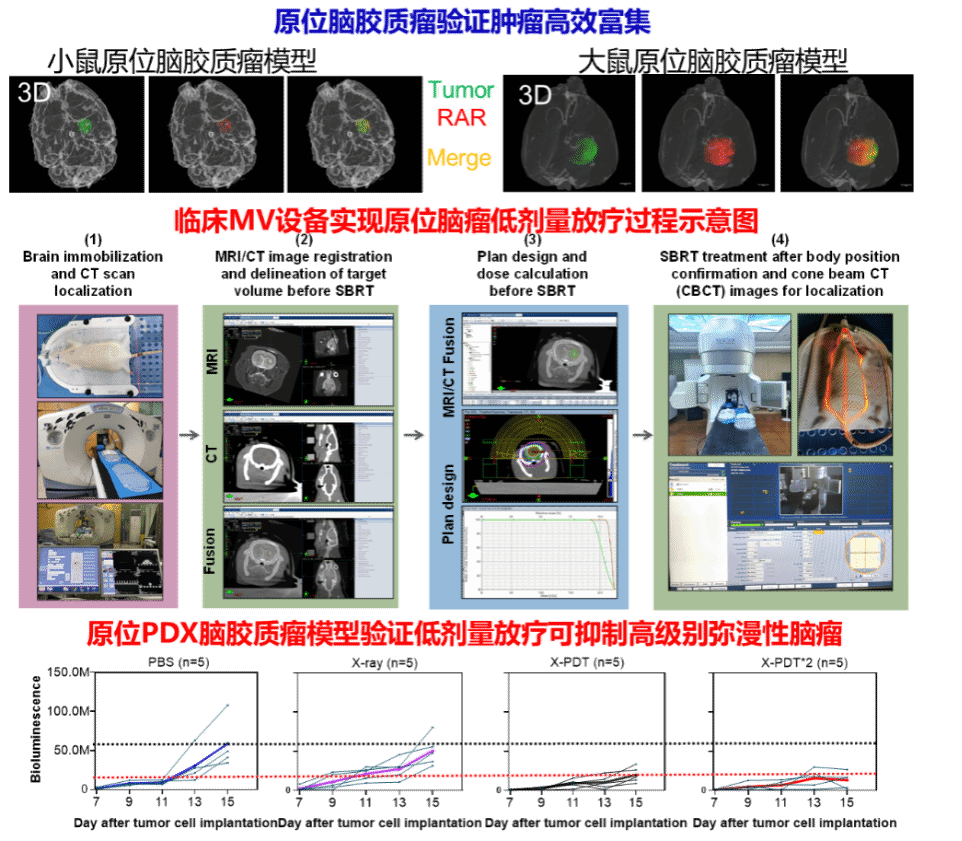
The diffuse and infiltrative nature of high-grade gliomas poses ongoing challenges in treatment and management. Radiotherapy is an important glioma treatment, with a standard radiotherapy dose of 60 gray. However, high-dose radiotherapy is associated with radiation-induced side effects on normal tissue. Scintillator-mediated low-dose x-ray–induced photodynamic therapy using kilovoltage x-rays has been shown to be effective for multiple tumor types without causing damage to healthy tissue. However, x-ray–induced photodynamic therapy is yet to be explored in glioma because of the inability of the photosensitizers to cross the blood-brain barrier. Here, we present an integrated gold clustoluminogen containing protein-protected gold nanoclusters conjugated to a photosensitizer and a cell-penetrating peptide. Using intravital imaging, we showed that gold clustoluminogen crossed the intact blood-brain barrier in healthy animals and accumulated in tumors in two murine intracranial orthotopic glioma models. Gold clustoluminogen efficiently suppressed glioma growth and prolonged animal survival under ultralow-dose x-ray treatment (total 2 gray, megavoltage x-ray) using the same protocol as that used for clinical megavoltage radiotherapy. Moreover, gold clustoluminogen potently inhibited tumor growth in an orthotopic patient-derived xenograft glioma model with prolonged animal survival under ultralow-dose x-ray treatment. Gold clustoluminogen was eliminated through hepatic and renal excretion, with no observed toxicity. These results highlight new opportunities to develop clinically relevant glioma therapies with reduced side effects.
Link:https://www.science.org/doi/10.1126/scitranslmed.adq5331