Abstract
Background Globally, an estimated 296 million individuals live with chronic hepatitis B virus (HBV) infection, carrying substantial risks of liver fibrosis, cirrhosis and hepatocellular carcinoma. Fewer than 20% of patients receiving nucleos(t)ide analogues or interferons achieve a functional cure, underscoring the urgent need for novel therapeutic strategies to improve clinical outcomes in patients with chronic HBV infection.
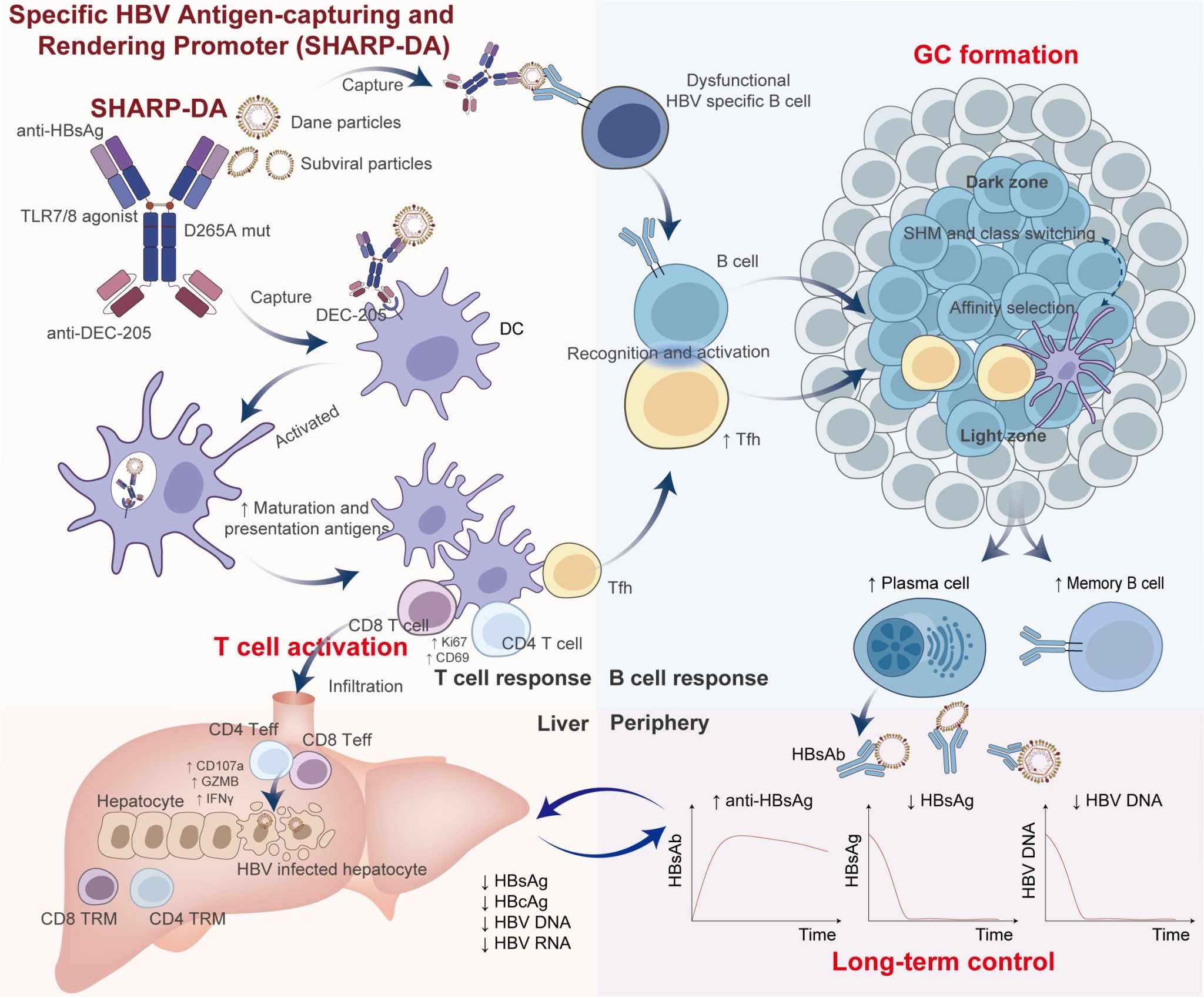
Objective The aim of this study was to develop a ‘virus-hunter vaccine’ that hijacks HBV antigens as endogenous immunogens, reprogramming dendritic cells (DCs) to prime anti-HBV immunity, ultimately achieving a durable functional cure beyond current therapeutic limitations.
Design We engineered the SHARP (Specific HBV Antigen-capturing and Rendering Promotor) vaccine platform, comprising a bispecific antibody targeting hepatitis B surface antigen (HBsAg) and DEC-205, conjugated with toll-like receptor 7/8 agonists. Therapeutic efficacy was assessed in chronic HBV carrier mice, with comprehensive investigation of immunological mechanisms.
Results Both SHARP variants demonstrated enhanced antigen phagocytosis, maturation and antigen presentation of DCs. Notably, SHARP-D265A (DA) emerged as the lead candidate due to its optimised Fc silencing, showing superior therapeutic efficacy with a lower anti-drug antibody incidence. SHARP treatment reversed the tolerogenic microenvironment through coordinated activation of HBV-specific CD4+ and CD8+ T cells and established durable viral control: HBsAg was below the limit of detection, accompanied by the appearance of anti-HBsAg, which was maintained for more than 161 days with established immune memory against rechallenge.
Conclusion This innovative HBV vaccine strategy actively captures viruses, overcoming the tolerogenic immune microenvironment of chronic HBV infection, offering a novel strategy for the treatment of chronic HBV infection and other immune-tolerant diseases.
Link:https://gut.bmj.com/content/early/2025/11/05/gutjnl-2025-335806