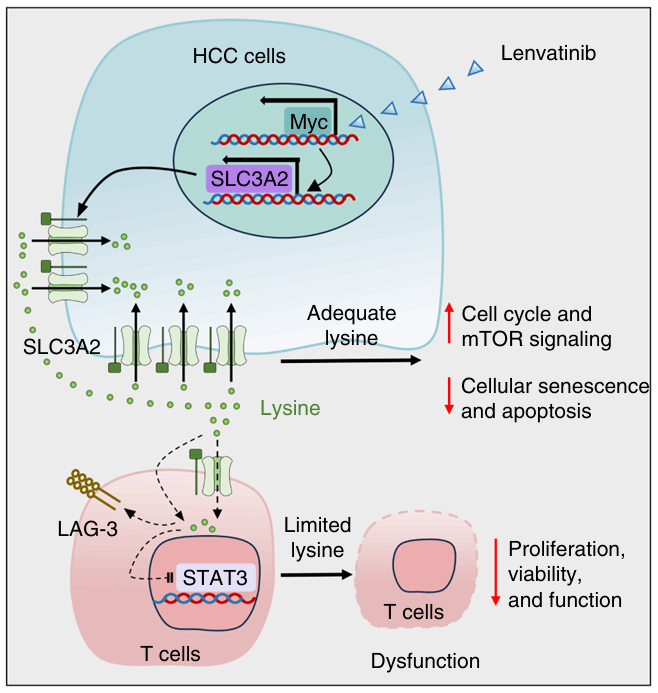
Abstract
Abnormal amino acid metabolism supports cancer cell proliferation, invasion, and immune evasion in hepatocellular carcinoma (HCC). Previous research exploring amino acid metabolism in HCC has primarily focused on how metabolic reprogramming impacts tumor cells. Here, we focused on the role of amino acid metabolism dysregulation in the crosstalk between HCC and T cells. HCC cells disrupted lysine uptake in T cells, leading to impaired T cell immunity. Lysine deprivation decreased STAT3 levels in T cells, inhibiting T cell proliferation and effector function and ultimately promoting tumor progression. Mechanistically, HCC cells outcompeted T cells for lysine by expressing high levels of the lysine transporter SLC3A2. Clinically, elevated SLC3A2 expression correlated with poor survival and was linked to dysregulated T cell functional gene signatures in HCC patients. Furthermore, the multikinase inhibitor lenvatinib induced a c-Myc-SLC3A2 regulatory axis that limited the efficacy of lenvatinib treatment. Lysine supplementation enhanced tumor sensitivity to combined treatment with lenvatinib and anti-PD-1 immunotherapy. These findings suggest that lysine supplementation is a potential therapeutic strategy for treating HCC and enhancing the sensitivity of HCC to tyrosine kinase inhibitors and immune checkpoint blockade.
Link: https://aacrjournals.org/cancerres/article-abstract/doi/10.1158/0008-5472.CAN-24-3180/754363/SLC3A2-Mediated-Lysine-Uptake-by-Cancer-Cells?redirectedFrom=fulltext