Abstract
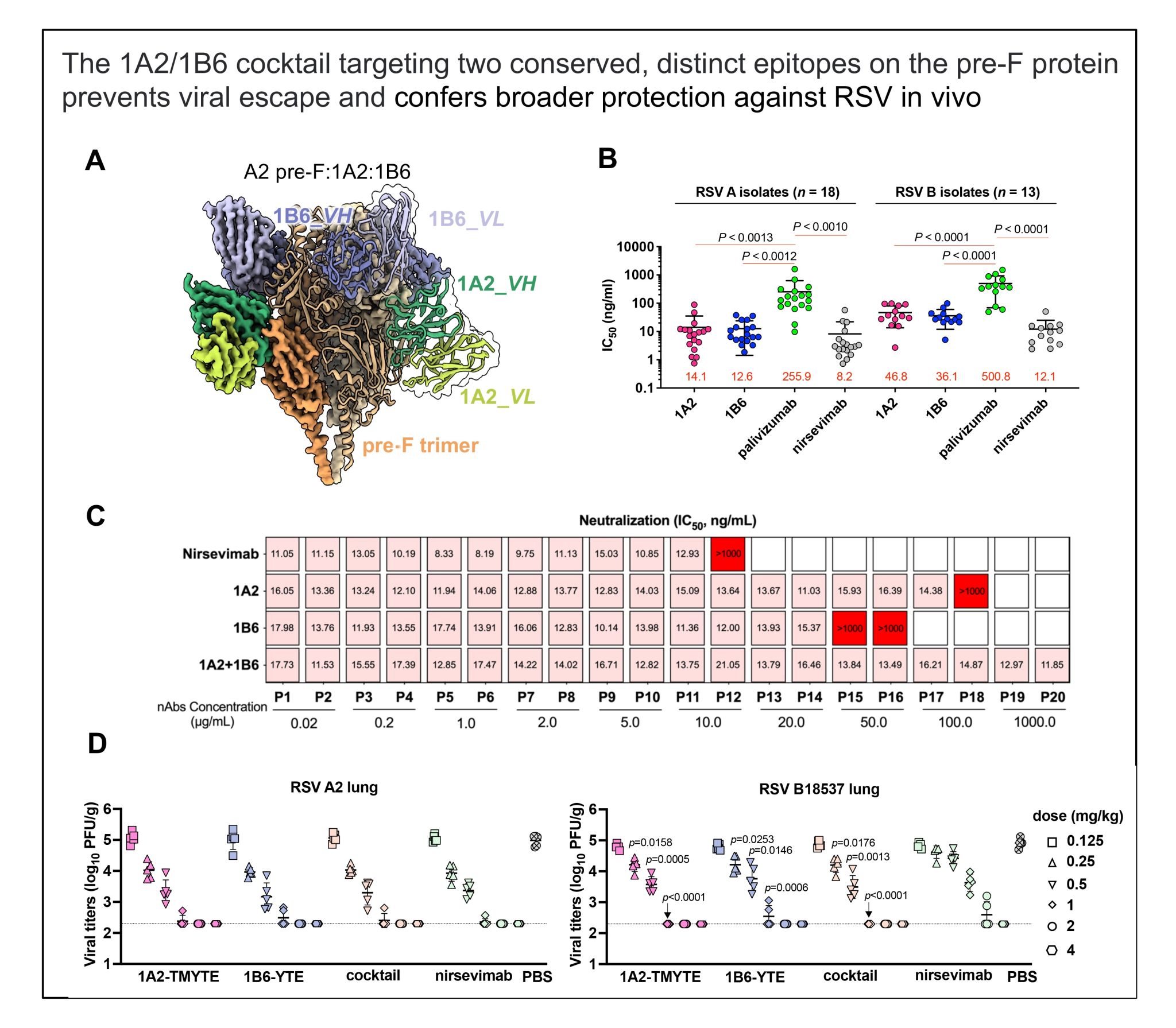
Respiratory syncytial virus (RSV) poses a critical threat to infants, yet vaccine and antibody development remains challenged by safety risks and antigenic variability. Here, we present a prophylactic strategy leveraging two human neutralizing antibodies, 1A2 and 1B6, which target distinct, conserved epitopes on the RSV prefusion F (pre-F) protein. Cryo–electron microscopy (cryo-EM) structural analysis revealed that 1A2 binds a “waist” epitope spanning antigenic sites IV/V, whereas 1B6 engages a “head” epitope bridging sites Ø/II/V, collectively stabilizing the pre-F trimer to block conformational transitions critical for viral entry. In vitro escape mutagenesis demonstrated that the 1A2/1B6 cocktail can resist viral escape (>20 passages), contrasting with rapid resistance to nirsevimab (targeting site Ø) and single antibodies [1A2: Gly446→Glu (G446E); 1B6: Gln94→Arg (Q94R) or Gln94→Lys (Q94K)]. Fc engineering extended serum half-lives while ablating effector functions, addressing potential safety concerns. Last, prophylactic administration in rodent models conferred robust protection against RSV A and B strains, including nirsevimab-resistant variants, with a 296-fold reduction in lung viral titers. This dual-epitope approach overcomes limitations of current monotherapies by combining high conservation, synergistic potency, and escape resilience, positioning it as a valuable immunoprophylactic candidate for pediatric RSV prevention.
Link:https://www.science.org/doi/10.1126/scitranslmed.ady2450